Formlabs Biomed Amber Resin
BioMed Amber Resin
Formlabs developed BioMed Amber Resin specifically for nasopharyngeal (NP) swabs used in diagnostic testing. During the COVID-19 pandemic, there was a nationwide shortage of NP swabs in the United States. These swabs are typically used for testing influenza or other respiratory infections. The shortage became so severe that clinicians began designing and testing their own swabs, prioritizing speed and safety.
BioMed Amber Resin is a strong, rigid material suitable for biocompatible applications that involve long-term skin contact (>30 days), or short-term contact with bone, tissue, dentin, or mucosa (<24 hours). Parts printed with BioMed Amber Resin are compatible with common solvent disinfection and sterilization methods. This resin is manufactured in our FDA-registered, ISO 13485-certified facility.
*If the product is in stock, it will be delivered within 3 days after the order is placed. If the product is out of stock, delivery will be made within 3 days after restocking.
A New Medical Material Developed by Formlabs! — BioMed Amber Resin
[Application of BioMed Amber Resin in Swab Production]
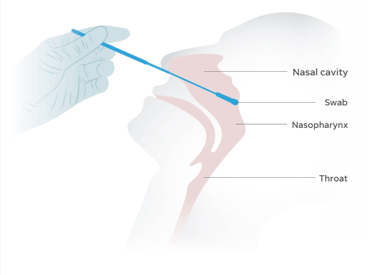
What Is a Nasopharyngeal Swab?
Challenge:
During the COVID-19 pandemic, the United States faced a nationwide shortage of nasopharyngeal (NP) swabs needed for testing. These swabs are commonly used for influenza or other respiratory infection diagnostics. The shortage became so severe that clinicians began designing and testing swabs themselves—urgently, safely, and speedily.
A nasopharyngeal swab is a soft-tipped stick with bristles at the end that is inserted into the back of the nasal cavity and rotated to collect mucus samples. The swabs are then placed into vials containing transport media. 3D-printed NP swabs are designed with a break point 7–8 cm from the tip to retain the sample end and fit securely into the vial for transport to the testing lab.

How Are NP Swabs Made?
Solution:
Upon recognizing the critical shortage of NP swabs required for COVID-19 testing, the 3D applications team at USF Health created an initial design. Working in collaboration with Northwell Health and Formlabs, they developed an innovative, safe material to serve as a 3D-printable alternative. Within just one week, the team produced swab prototypes and began testing at USF Health and Northwell Health. Two days later, both institutions continued development using Formlabs 3D printers and this biocompatible, high-pressure sterilizable resin. To ensure optimal safety and comfort for patients, swabs were tested at USF Health, Northwell Health, and Tampa General Hospital. Clinical validation is now complete, and 3D printing at USF Health and Northwell Health is underway to supply swabs to patients.
Validation Status
The team overcame all testing challenges and successfully produced nasopharyngeal swabs. A critical milestone was achieved through validation tests (24‑hour, 3‑day, therapeutic) conducted by researchers at USF Health in collaboration with Northwell Health’s Department of Radiology and Infectious Diseases, followed by rapid clinical trials at Northwell Health and Tampa General Hospital. All results demonstrated that the 3D-printed NP swabs performed as well as standard swabs in detecting COVID-19.
These NP swabs are classified as Class I medical devices, exempt from premarket notification, though manufacturers must register them. Formlabs plans to produce swabs at its FDA-registered, ISO 13485-certified facilities.
Global Response
“This is a remarkable example of how academic experts, healthcare systems, and the tech industry can collaborate to make a real impact on people’s lives. In the current COVID-19 crisis, we cannot waste any time, and rapid, coordinated action has significantly increased national testing capacity.”
— Charles J. Lockwood, MD, MHCM, Senior Vice President for USF Health and Dean of USF Health Morsani College of Medicine
“This gave us hope and enabled us to face the pandemic proactively. Without testing, we cannot isolate effectively.”
— Dr. Goldstein, Director of 3‑D Design & Innovation, Northwell Health, Manhattan
“Although the unit cost is slightly higher, the ability to get deliveries within the week instead of weeks is a critical advantage.”
— Terry Wohlers, President of Wohlers Associates Inc.
Disclaimer
During this global health emergency, Formlabs created this site and participated in various initiatives. While helping alleviate medical supply shortages, patient safety remains Formlabs’ top priority. It is important to note that 3D‑printed masks, swabs, face shields, and other items used in COVID‑19 response are medical products. These products must be safe, and if you choose to produce them, please consider the following:
- Formlabs is a materials manufacturer, and printed end‑products must be validated and verified for their intended purpose based on material labeling.
- The end‑product you create may not yet have regulatory approval. If producing finished medical devices, follow all labeling guidelines. You may seek professional regulatory advice.
- Before manufacturing medical devices with 3D printing, consult regional regulations, material safety data, software capabilities, sterilization requirements, and control protocols.
- Regulatory bodies (e.g., FDA) may require submission of manufacturing information and/or pre‑market documentation.
Formlabs cannot guarantee that products not made by Formlabs will meet intended use requirements.
Regulatory Statement
Under 21 CFR §880.6025, absorbent tipped applicators used to collect patient samples for nasopharyngeal swabs are classified as Class I medical devices and are exempt from premarket notification. FDA requires medical device manufacturers to register their facilities and list their products under 21 CFR §807.20.
Formlabs facility registrations: Formlabs Inc. – Registration No. 3010279788 Formlabs Ohio Inc. – Registration No. 3015491441 Formlabs 3D‑printed swabs are listed under FDA registration No. 656158 (KXF).
Further Reading:
1. In Taiwan, many users are combining 3D printing and medical know‑how to produce PPE items such as protective goggles, face shields, and mask comfort straps.
TEAMA × Atom × Medical Fablab NDMC livestream sharing pandemic‑defense project—tune in with us.
2. Exclusive interview on FTV News with the NDMC Medical 3D Printing Center team.
Interested in learning more about 3D printing applications in healthcare or collaborating with the NDMC Medical 3D Printing Center?
Fill out the form below to let us know what you'd like to learn—we'll provide you with the most comprehensive solutions!

 (
(